Immunization Action Coalition IAC. Thats why all of our supplements are made in America in NSF-certified and FDA-inspected facilities that operate in accordance with the Current Good Manufacturing Practice cGMP regulations.

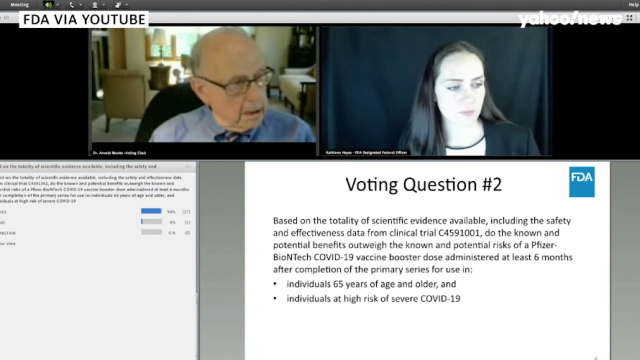
Fda Advisors Reject Pfizer Covid 19 Boosters For All But Agree To Shots For Elderly And High Risk Fiercepharma
Boostrix by GSK was licensed for use as an active booster in persons 10-18 years of age.

Fda booster recommendation. There are two reasons that FDA could go against the recommendation Stifel analyst Paul Matteis wrote in a Dec. While most Wall Street analysts doubt the FDA will approve the drug some are holding out the possibility of a surprise by the decision deadline of March 7. In addition the FDA approval ignored the recommendation of its outside advisors who said Biogen did not provide enough evidence of clinical benefit.
1004 PM PDT June 30 2021. BioVaxys Technology Corp. Precision Vaccinations Johnson Johnson JJ announced it welcomes the recommendation by the World Health Organization WHO Strategic Advisory Group of Experts on Immunization for the Janssen Ebola vaccine regimen Zabdeno Ad26ZEBOV and Mvabea MVA-BN-Filo.
An allergic reaction could occur after the vaccinated person leaves the clinic. This recommendation supersedes previous Tdap recommendations regarding adults aged 65 years and older. FDAs guidance documents including this guidance do not establish legally enforceable.
May 3 2005 An acellular pertussis vaccine combined with the adult formulation of tetanus and diphtheria Tdap. Despite questions surrounding its efficacy the Food and Drug Administration on Monday approved a groundbreaking new medication that attacks the underlying Alzheimers disease process rather than treating just its symptoms writes The New York TimesIt is the first drug of its kind and the first new Alzheimers treatment in 18 years. I support the ACIPs recommendation that the Johnson and Johnson COVID-19 vaccine be used for persons 18 years of age or older in the United States population under the FDA emergency use.
Three of the advisory panels members have. BVAXF BioVaxys is pleased to announce today that the US Food and Drug Administration FDA has reviewed its Pre-IND request for a. FDA licensed a 2nd Tdap vaccine Adacel by Sanofi Pasteur for use in persons ages 11-64 years.
If you see signs of a severe allergic reaction hives swelling of the face and throat difficulty breathing a fast heartbeat dizziness or weakness call 9-1-1 and get the person to the nearest hospital. But no specific recommendation. Zabdeno is given first and Mvabea is administered.
The Pertussis Vaccines Work Group of ACIP reviewed the epidemiology of pertussis in adults aged 65 years and older and two cost-effectiveness models to assess the epidemiologic and economic impact of pertussis vaccination in this population. Health experts say that any recommendation about booster shots should be made by public health officials at the CDC and FDA and not by pharmaceutical executives. Health regulator this year for its Alzheimers candidate weeks after a rival drug from Biogen Inc won the agencys.
Regulators are slated to decided by Monday whether to approve Biogen Incs controversial Alzheimers disease drug and Wall Street analysts and industry observers are deeply divided on its chances of making it over the finish line. The FDA has now given the drug the all-clear. If approved Biogens aducanumab would be the first treatment to address an underlying cause of the memory-robbing condition which is the sixth leading cause.
17 note to clients. There is still no federal public health recommendation for COVID-19 booster shots. We pay a premium to work with the best manufacturers in the country but its the only way we can also produce the best all-natural sports supplements.
The FDA on Monday gave the drug accelerated approval based on evidence that it can reduce a likely contributor to Alzheimers rather than proof of a clear benefit against the disease. Timing of dosing primary and booster schedule and dosage range. All recommendations about booster shots need to come from FDA and the CDC not the.
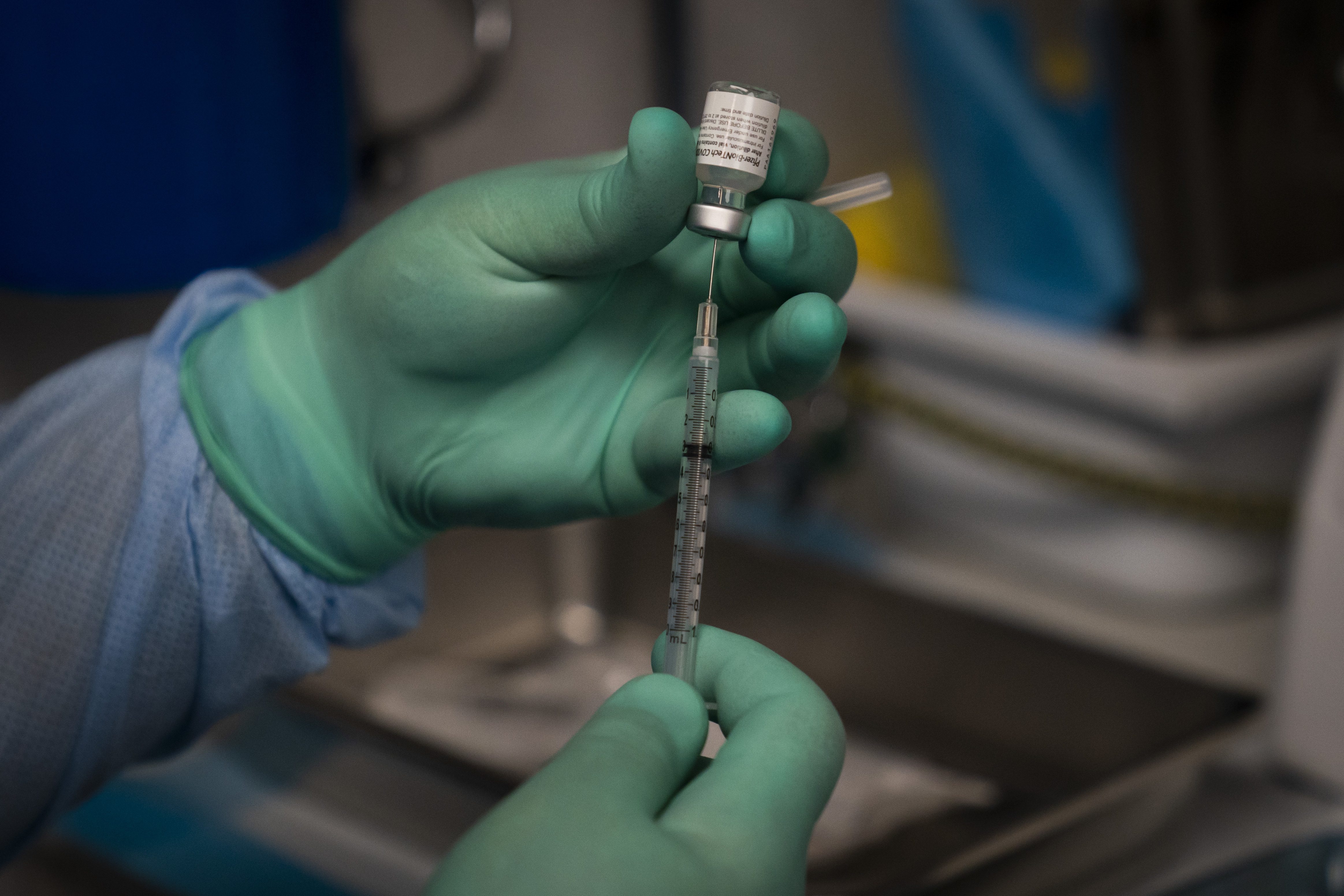
A booster dose of mRNA-1273351 the Companys strain-matched booster achieved higher neutralizing antibody titers against the B1351 variant of concern than a booster dose of mRNA-1273. The Janssen Ebola vaccine therapy consists of two components. Eli Lilly and Co said it will seek accelerated approval from the US.
The FDA has not weighed in on mixing brands of COVID-19 vaccines but that has not stopped some doctors from recommending the practice. For other signs that concern you call your health care provider. In trials the drug in combination with diet and exercise helped patients lose over 12 more weight than those who took a placebo along with.

Fda Panel Recommends Booster For Certain Groups Not General Public Supertalk Mississippi

Fda Recommends Covid 19 Booster Shots For Some Americans
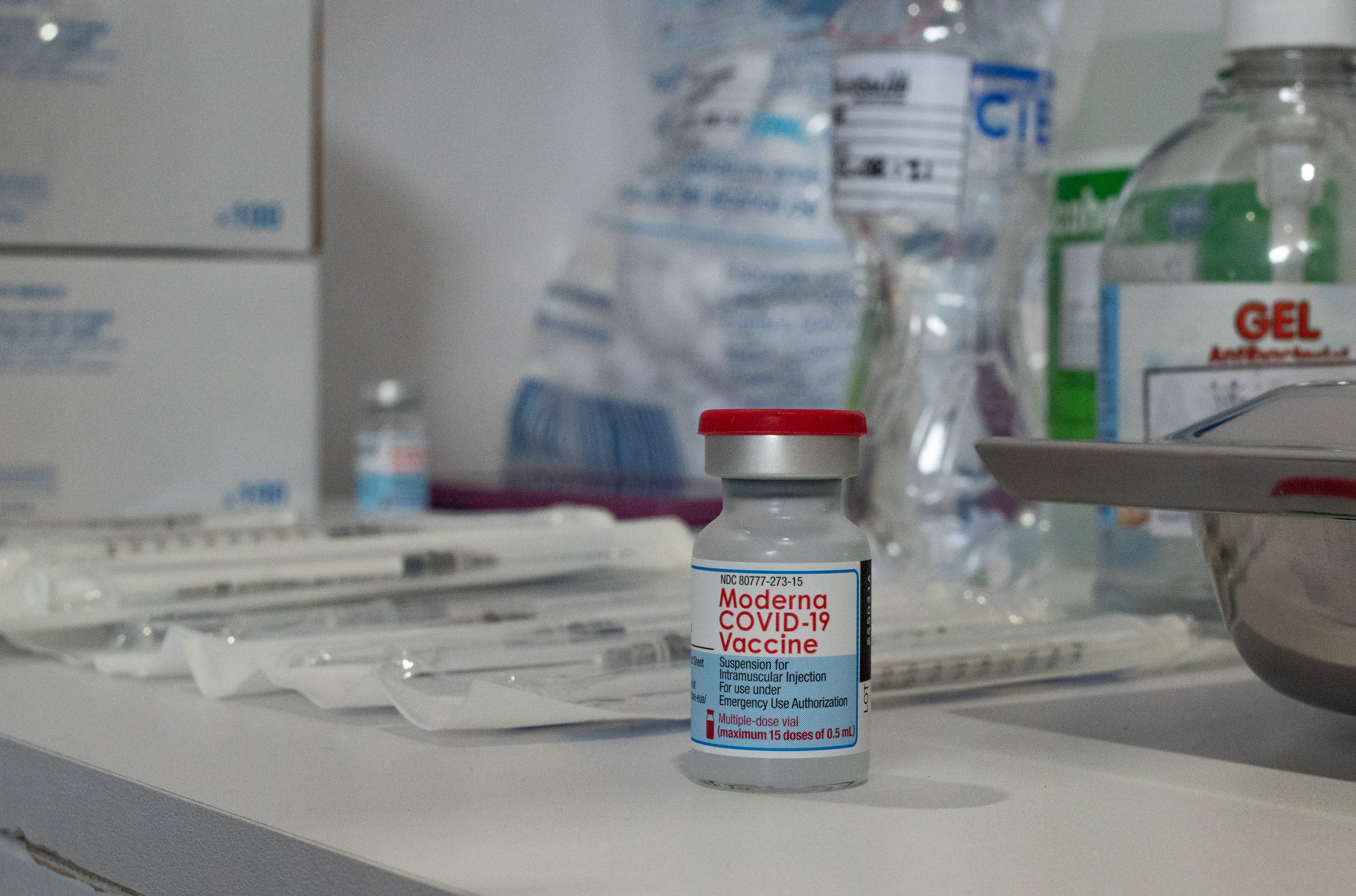
Moderna Asks Fda To Authorize A Booster Shot Of Its Covid 19 Vaccine Coronavirus Updates Npr

Pfizer Booster Shots Recommended Only For 65 After Fda Advisory Meeting
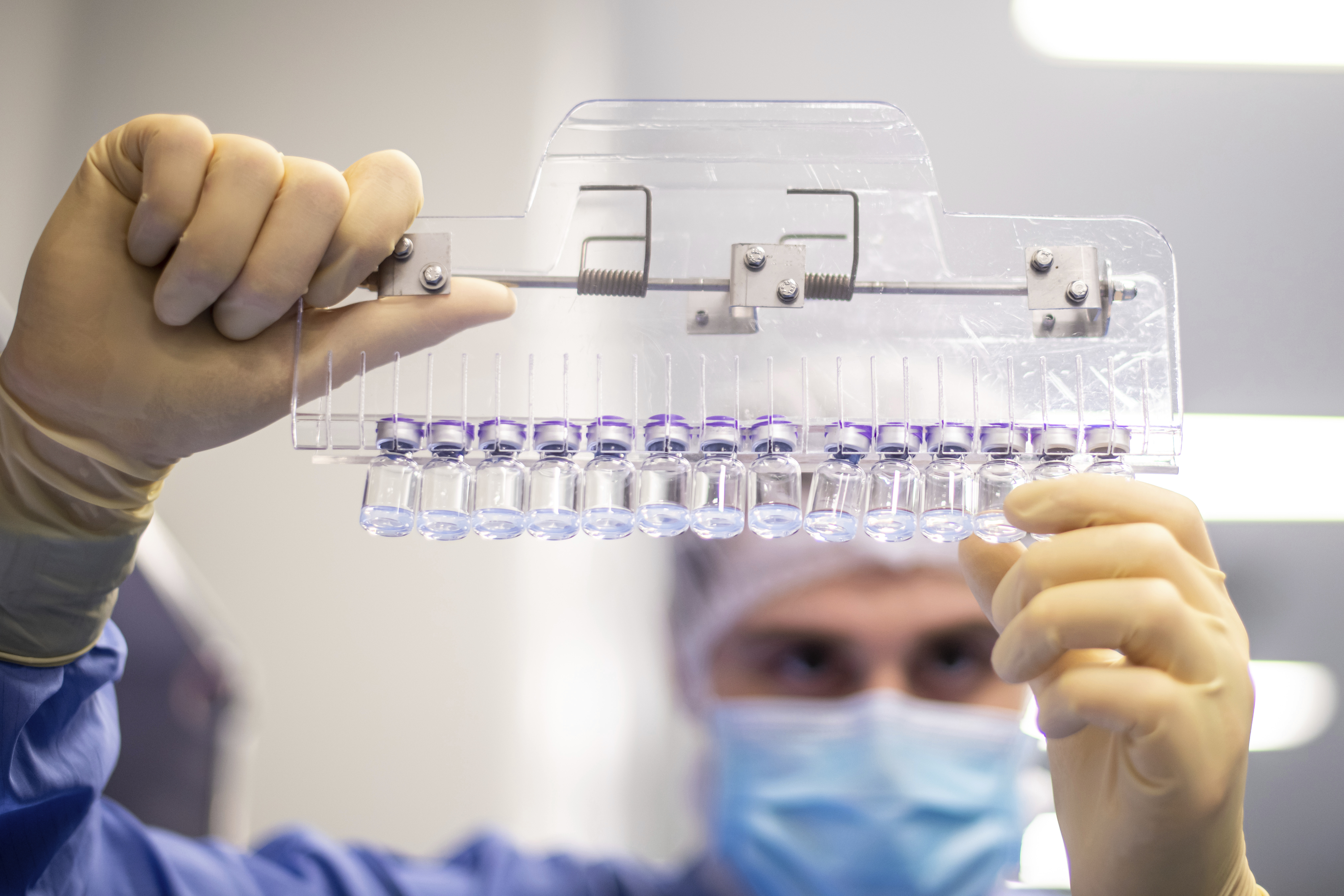
Fda Panel To Discuss Booster Shots Today Here S What You Need To Know The Boston Globe
Oregon Health Authority Issues Statement On Fda Booster Dose Recommendation Ktvz