11 2020 for administration in individuals 16 years of age and older. But a CDC advisory panel where the FDA made the announcement still strongly backs the vaccines benefits which outweigh the rare risk for heart inflammation.
NIH Chief Mulls Precision Booster Shots.

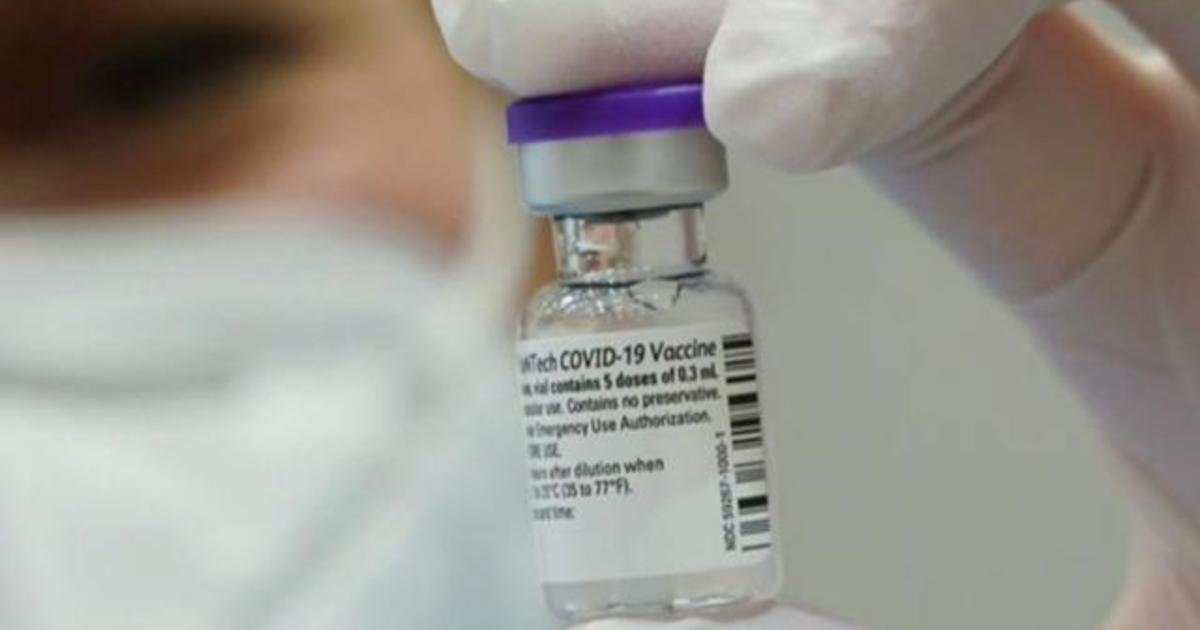
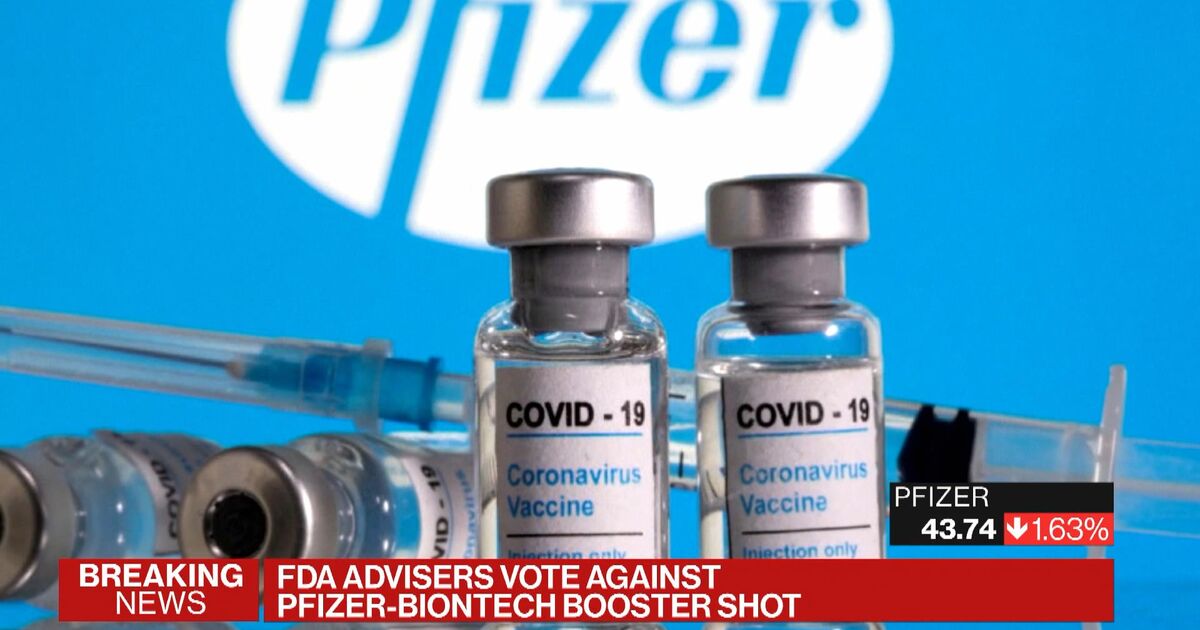
Fda booster vote. The US Food and Drug Administration FDA is adding a warning to the fact sheets for the PfizerBioNTech and Moderna mRNA COVID-19 vaccines as medical experts continue to investigate cases of heart inflammation which are rare but are more likely to occur in young men and teen boys. Americas Largest Teachers Union to Vote on Mandatory COVID Vaccinations Masks and Testing for Students More. Pfizer CEO says a COVID-19 vaccine booster or 3rd dose likely within 12 months.
Biogen shares were trading near 271 on Thursday down from their 52-week high of 35563 reached in November before the advisory committee vote. June 24 2021 -- The US. The Defenders COVID NewsWatch provides a roundup of the latest headlines related to the SARS CoV-2 virus including its origins and COVID vaccines.
The drugmaker says vaccine efficacy was 100 in its Phase 3 clinical trials. Michele Cohen Marill June 23 2021. A key element Pfizer and Moderna need to pinpoint in their trials is the specific measurable marker in the immune system called correlates.
The Washington DC City Council on Tuesday voted 8-5 to approve a bill to ban flavored tobacco products including those containing menthol. Moderna Pfizer and Johnson Johnson are all researching the potential use of. McClellan the former FDA commissioner said the United States excess supplies of the shot exist in case of complications such as emerging variants or demand for a booster shot and that.
If the FDA approves aducanumab Biogen could go to circa 400 and if not to circa 200 Mizuho Securities analyst Salim Syed said in a recent research note. 11 new deaths in Louisiana reported from Ida raising states death toll to. Even then however there may only be certain people who need booster shots such as.
Vaccine makers are preparing for a next possible phase of the Covid-19 vaccine rollout. EL PASO Texas KVIA As more Americans get the Covid-19 vaccine the race to research booster shots continues. In early April Pfizer and its partner BioNTech filed a request with the FDA to amend the emergency use authorization EUA to lower the eligibility age to 12.
Biogens aducanumab for Alzheimers The FDA is facing a no-win decision on Biogens Alzheimers treatment aducanumab. Sanjay Gupta reports on these booster studies. After collaborating with the company on review of its approval application in spite of mixed and controversial data a panel of outside advisers voted decisively against the drugs benefit-risk profile.
The FDA amended the emergency use authorization originally issued on Dec. If the FDA approves aducanumab Biogen could go to circa 400 and if not to circa 200 Mizuho Securities analyst Salim Syed said in a recent research note. Vote 2020 Texas.
Food and Drug Administration FDA expanded the emergency use authorization for the Pfizer-BioNTech COVID-19 Vaccine for the prevention of COVID-19 to include adolescents 12 through 15 years of age. Doran Fink MD PhD deputy director of the FDAs Division of. By edhat staff Today the US.
Russian President Vladimir Putin self-isolating due to coronavirus among inner circle AP. FDA liaison representative Doran Fink MD PhD noted the agency will add a warning about the risk of myocarditis or pericarditis following vaccination that. Food and Drug Administration FDA is adding a warning to the fact sheets for the PfizerBioNTech and Moderna mRNA COVID-19.
Story continues Biogen shares were trading near 271 on Thursday down from their 52-week high of 35563 reached in November before the advisory committee vote. Former Minneapolis police officers accused of violating George Floyds rights to be arraigned AP. World Health Organization chief urges halt to booster shots for rest of the year By KEVIN McGILL and MELINDA DESLATTE.
Which could mean booster shots would be recommended down the road. Pfizer said all of the subjects are being randomized into three groups one with the 20vPnC plus Pfizer-BioNTech COVID-19 vaccine booster which is a. Advances in precision medicine may help transform Covid-19 booster shots from a community-wide effort to one thats tailored to individual immune responses.
Fda Sounds Skeptical Note On Pfizer Booster Shot Ahead Of Key Vote Politico
Fda Panel To Discuss Booster Shots Today Here S What You Need To Know The Boston Globe

/do0bihdskp9dy.cloudfront.net/09-17-2021/t_482efbcf87dd4f6fbc792baa8659bf7b_name_file_1280x720_2000_v3_1_.jpg)